Thin-layer chromatography (TLC) is a widely used analytical technique in chemistry and biochemistry that allows the separation, identification, and quantification of different compounds in a mixture. It is a type of chromatography in which a stationary phase, typically a thin layer of an adsorbent material, is coated on a flat, inert support such as glass, aluminum, or plastic.
Here's a more detailed breakdown of the components and processes involved in thin-layer chromatography:
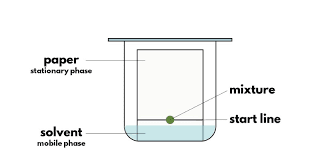
1. Stationary Phase: The stationary phase is a thin layer of an adsorbent material, most commonly silica gel or alumina, although other materials like cellulose or bonded phases can be used depending on the specific application. The adsorbent is chosen based on the types of compounds to be separated and their interactions with the stationary phase.
2. Mobile Phase: The mobile phase is the solvent or mixture of solvents that moves over the stationary phase, carrying the sample mixture along with it. The choice of the mobile phase is crucial since it determines the extent to which the compounds in the sample will interact with the stationary phase, affecting the separation.
3. Sample Application: A small spot of the sample mixture to be analyzed is applied near the bottom of the TLC plate using a capillary tube or a microsyringe. It's essential to apply the sample as a small, concentrated spot to ensure clear separation and accurate results.
4. Developing the Plate: The TLC plate is placed in a closed container, known as a development chamber, where the mobile phase rises up the plate through capillary action. As the mobile phase moves over the stationary phase, it interacts with the sample components, causing them to move up the plate at different rates based on their affinities for the stationary and mobile phases.
5. Chromatogram Visualization: After the mobile phase has traveled a specific distance (known as the solvent front), the TLC plate is removed from the chamber. The plate is then dried, and the separated components, now forming distinct spots along the plate, are visualized. Various techniques can be used for visualization, such as using UV light, iodine vapors, or specific chemical reagents that react with the separated compounds to produce colored spots.
6. Rf Value: The Rf (retention factor) value is a numerical measure used to identify and characterize the separated compounds. It is the ratio of the distance traveled by a compound from the baseline to the center of the spot (solvent front) to the distance traveled by the solvent front. Each compound has a characteristic Rf value under specific TLC conditions, and this value can be compared with known standards to aid in identification.
Applications of TLC:
- Analyzing the purity of chemical substances.
- Identifying components in mixtures.
- Monitoring the progress of chemical reactions.
- Testing for the presence of specific compounds.
- Separation of closely related compounds in natural products and pharmaceuticals.
Thin-layer chromatography is a quick, cost-effective, and simple technique with a wide range of applications in various fields, including chemistry, biochemistry, pharmacy, and environmental analysis. It is commonly used as a preliminary step before more advanced chromatographic techniques, such as high-performance liquid chromatography (HPLC) or gas chromatography (GC).




No comments:
Post a Comment